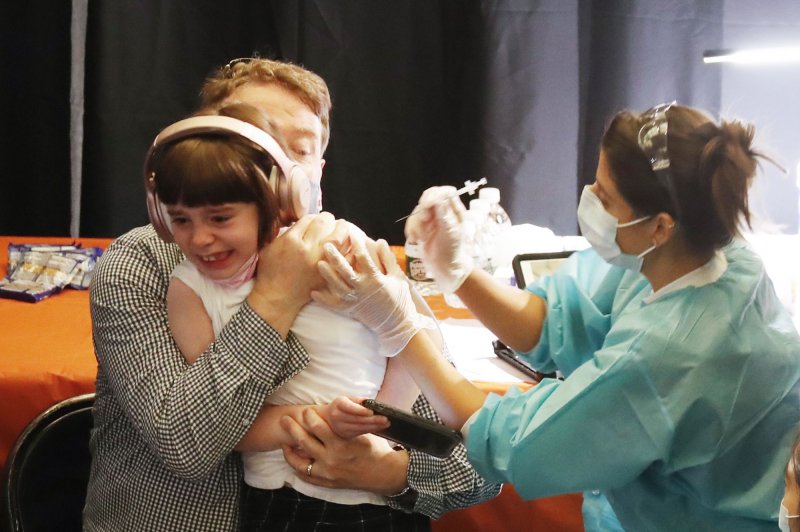
Nov. 25 (UPI) — The European Union’s health regulatory agency on Thursday issued approvals the use of the Pfizer-BioNTech COVID-19 vaccine for children aged 5 to 11.
The European Medicines Agency approved lower doses of the vaccine for younger children than those used for children ages 12 and up. The vaccine will still be given in two injections three weeks apart.
Studies cited by the EMA said common side effects included pain at the injection site, tiredness, headache, redness and swelling, and muscle pains. The side effects are usually mild and improve within a few days, the regulators said.
The efficacy of the vaccine is at 90.7%, according to a study of 2,000 children who had no previous infection.
Ireland won’t expect doses of the vaccine until Dec. 20, and the vaccine will likely be administered to school children before the end of the year, health officials said. The move will help Ireland’s National Immunization Advisory Committee to consider the full rollout of the vaccine.
Canada approved the vaccine for the same age group five days ago, following the U.S. Centers for Disease Control and Prevention recommendations for all adults to receive booster shots.
Children became eligible for the lower dose vaccine in the United States earlier this month. Those who receive their shots are expected to be fully vaccinated by Christmas.